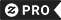Tap / click on image to see more RealViewsTM
Sale Price $24.87.
Original Price $29.25 Comp. value
per mug
You save 15%
Which one will dissolve in water Polar Chemistry Travel Mug
Qty:
Style
Travel/Commuter Mug
-$10.30
-$9.25
-$8.25
-$5.15
-$4.10
-$2.05
Size
Color
Stainless Steel
About Mugs
Sold by
About This Design
Which one will dissolve in water Polar Chemistry Travel Mug
Polar bears are soluble in water. Solubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a liquid solvent to form a homogeneous solution. The solubility of a substance fundamentally depends on the used solvent as well as on temperature and pressure. The extent of the solubility of a substance in a specific solvent is measured as the saturation concentration where adding more solute does not increase the concentration of the solution. The solvent is generally a liquid, which can be a pure substance or a mixture.[1] One also speaks of solid solution, but rarely of solution in a gas (see vapor-liquid equilibrium instead) The extent of solubility ranges widely, from infinitely soluble (fully miscible[2] ) such as ethanol in water, to poorly soluble, such as silver chloride in water. The term insoluble is often applied to poorly or very poorly soluble compounds. Under certain conditions the equilibrium solubility can be exceeded to give a so-called supersaturated solution, which is metastable
Customer Reviews
4.8 out of 5 stars rating21.8K Total Reviews
21,840 Reviews
Reviews for similar products
5 out of 5 stars rating
By AnonymousAugust 31, 2025 • Verified Purchase
Travel/Commuter Mug, 15 oz
everything is still going great .
5 out of 5 stars rating
By Suzanne H.January 28, 2025 • Verified Purchase
Travel/Commuter Mug, 15 oz
Creator Review
My husband loves his custom personalized Bible verse travel mug, here’s a screenshot of his Facebook post he shared on social media.
5 out of 5 stars rating
By Faith I.January 5, 2016 • Verified Purchase
Travel/Commuter Mug, 15 oz
Creator Review
Impressive stainless steel quality. Purchased as Christmas gift for someone. They loved having their name on it! Will buy again. Next time for graduation gift. Very pleased with imaging-legible text, colors crisp
Tags
Other Info
Product ID: 168632215263790115
Created on: 4/17/2010, 5:34 PM
Rating: G
Recently Viewed Items